The central theme of Dr. Lee’s research program is primarily on mechano-transduction via the application of biomechanical analysis and biomedical optical imaging principles and techniques. Using light-sheet and light-field microscopy, Dr. Lee’s lab is investigating the mechanisms whereby hemodynamics or cardiac contractiltiy regulates the development of the ventricular chamber. His group is linking the effects of biomechanical effects on developmental biology to understand the mechanisms of development and heart regeneration using the zebrafish animal model. The goal of our research is to translate our findings to higher levels of animals in collaboration with UT Southwestern Medical Center.
Contents
Hemodynamics and Cardiac Development
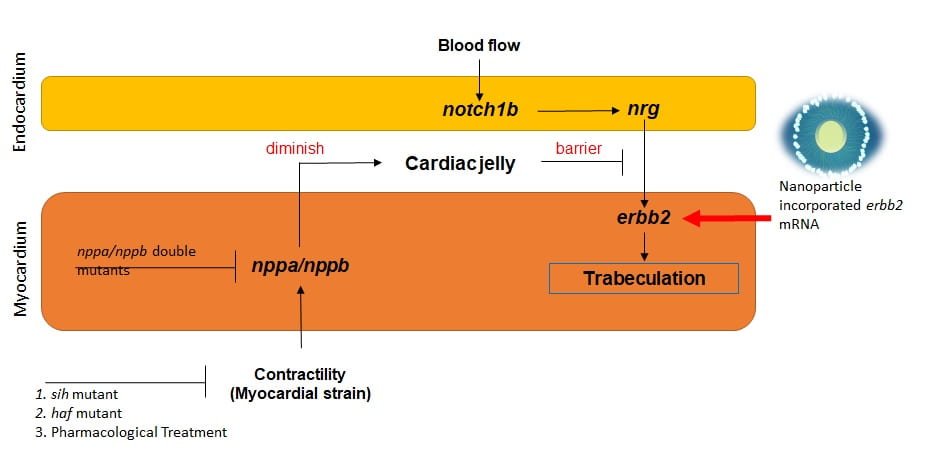
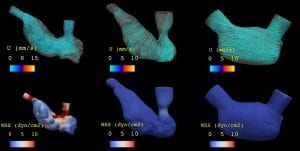
By taking advantage of the rapid development of zebrafish hearts, we assessed shear stress from blood flow affects ventricular morphology. My work focused on morphology of cardiac trabeculae under different blood flow conditions and by genetic manipulation of the Notch signaling pathway. In collaboration with Dr. Alison Marsden at Stanford University, we simulated and quantified intracardiac mechanical properties in 2-D. Currently, we are further working on the simulation of beating zebrafish hearts from light-sheet microscopy images with a 4-D synchronization algorithm based on voxel super-resolution.
Novel optical imaging techniques for Cardiac Imaging
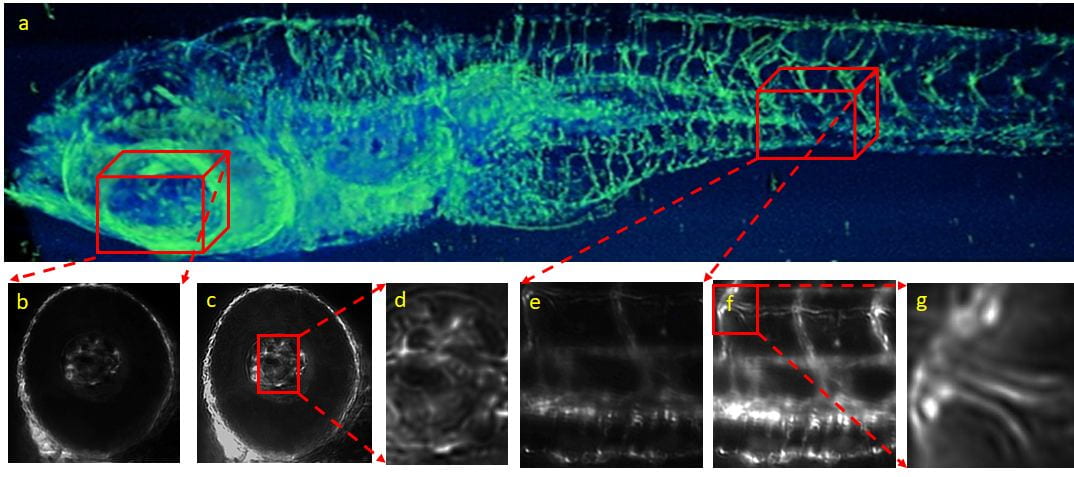
We built multiple light-sheet microscopy to image multi-scale cardiac imaging from micrometer to millimeter. Together with 4-D synchronization algorithm, beating zebrafish hearts were reconstructed, allowing measurement of cardiac mechanics. Our tunable light-sheet microscope with voxel super-resolution technique resolved a 5-times higher magnification than the original objective lens. We contributed that low magnification objective providing large field-of-view can capture detail structure of the samples.
Gene delivery or editing using PLGA nanoparticles
PLGA nanoparticle targeted delivery in collaboration with Dr. Kytai Nguyen and Dr. Zui Pan enable the over-expression of specific target gene for recovering the capability of cardiac tissue development or regeneration after modulation of biomechanical effects. Specifically, we aim to recovering Notch signaling inhibited by using of transgenic heat shock zebrafish model and engineered zebrafish by injecting nanoparticles loaded with a CRISPR/CAS9 system for deleting Notch ligands.
Cardiac Physiology in Cardiac Regeneration Process
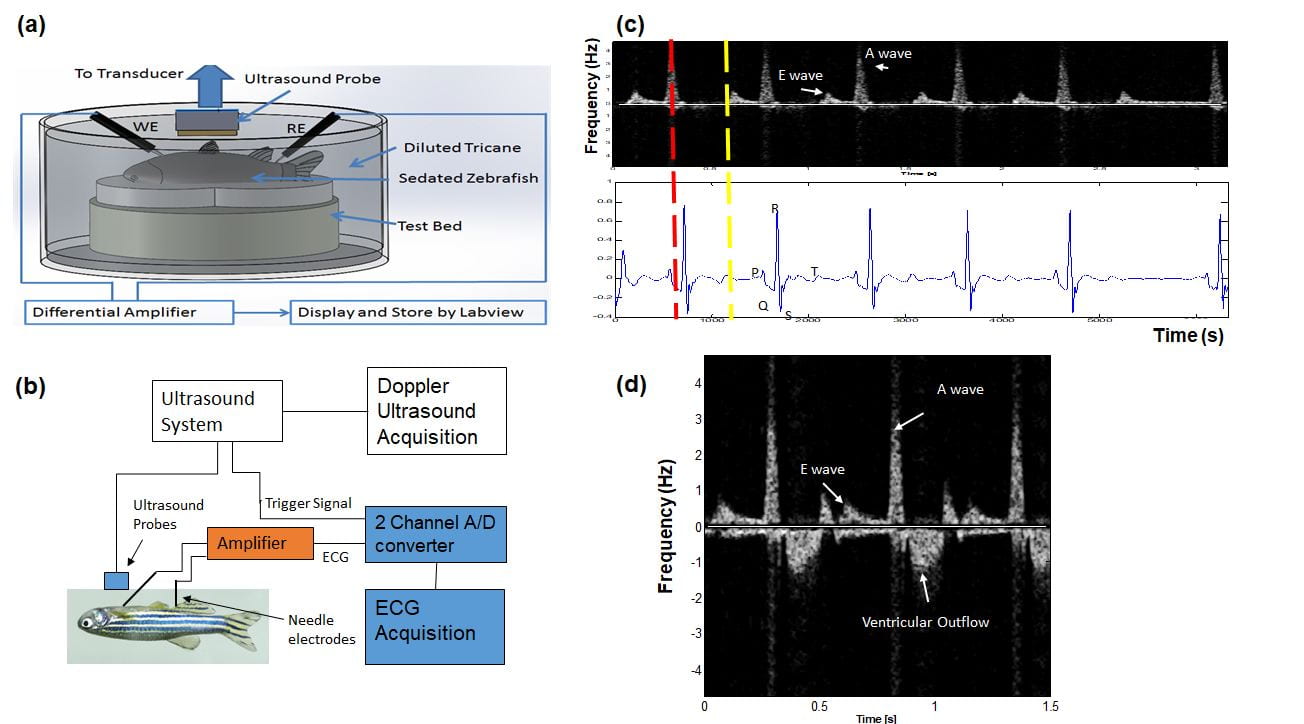
To identify the translational implications of cardiac regeneration in terms of physiology, we took advantage of cardiac regeneration in adult zebrafish after cryoinjury. Our micro-ECG device and micro-pressure measurement system in collaboration with Dr. Hung Cao at UC Irvine permit the monitoring of cardiac physiology during regeneration. PCR-array, immunostaining and light-sheet microscope help us visualize how the inflammatory process and structure can be recovered.